Yes, current research strongly supports the link between Type 1 Diabetes (T1D), gut dysbiosis, and low Short-Chain Fatty Acids (SCFAs). While T1D is an autoimmune condition, the “gut factor” is increasingly viewed as a critical environmental trigger that may turn on the genetic predisposition.
Here is a breakdown of the science regarding the connection and the potential for improvement.
1. Is Type 1 Diabetes a Sign of Gut Dysbiosis and Low SCFAs?
Yes. T1D is frequently accompanied by a distinct “microbial signature” characterized by low diversity and a specific lack of SCFA-producing bacteria.
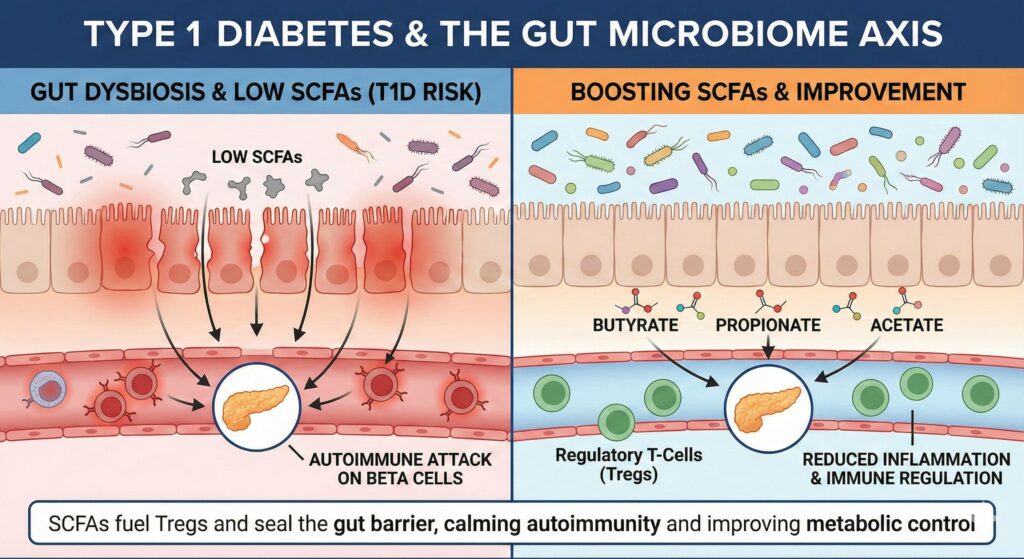
- The “Leaky Gut” Precursor: Research suggests that gut dysbiosis often precedes the clinical onset of T1D. A lack of butyrate (a primary SCFA) leads to a breakdown in the epithelial barrier (Leaky Gut).
- Molecular Mimicry: When the gut barrier is permeable, bacterial antigens leak into the bloodstream. Some of these antigens look structurally similar to the beta cells in the pancreas. The immune system attacks the bacteria but, due to confusion, also attacks the pancreas (autoimmunity).
- The Missing Peacekeepers: SCFAs, particularly Butyrate and Propionate, are essential for differentiation of Regulatory T-Cells (Tregs). Tregs are the immune system’s “peacekeepers” – they calm down autoimmune attacks. In T1D patients, SCFA levels are often critically low, leading to fewer Tregs and an uninhibited autoimmune assault.
2. Can Boosting SCFAs Improve Type 1 Diabetes?
Yes, but as a management strategy, not a cure. Boosting SCFAs aims to calm the immune system and improve metabolic control, rather than regenerating dead beta cells.
Mechanism of Improvement
- Halt Autoimmune Progression: By increasing butyrate, you increase the production of Tregs. In early-stage T1D (or the “honeymoon phase”), this could theoretically slow the destruction of remaining beta cells.
- Improved Insulin Sensitivity: Even in T1D, insulin resistance can occur (often called “Double Diabetes”). SCFAs (especially propionate) improve how the body utilizes the injected insulin, potentially stabilizing blood sugar levels and reducing insulin requirements.
- Reduced Inflammation: T1D is a state of chronic low-grade inflammation. SCFAs systematically lower pro-inflammatory cytokines (like IL-6), which protects other organs (kidneys, eyes, heart) from diabetic complications.
Research Spotlight: The HAMSAB Study
A significant human study involving a supplement called HAMSAB (High-Amylose Maize Starch Acetylated and Butyrylated) showed promising results.
- The Intervention: T1D patients were given a specialized resistant starch designed to deliver high amounts of acetate and butyrate to the colon.
- The Result: The patients saw a shift in their gut microbiome, higher SCFA levels in the blood, and—crucially—improved glycemic control and a more regulated immune profile.
Summary Table: SCFAs and Type 1 Diabetes
| SCFA | Role in T1D |
| Butyrate | Seals the gut barrier to stop antigen leakage; fuels Regulatory T-Cells (Tregs) to calm autoimmunity. |
| Propionate | Improves insulin sensitivity; reduces systemic inflammation that leads to diabetic complications. |
| Acetate | Feeds other beneficial bacteria (cross-feeding) to produce more butyrate; supports overall gut pH balance. |
While insulin manages the blood sugar, SCFAs manage the immune system behind the blood sugar. Restoring the gut microbiome isn’t about replacing insulin, but about protecting the body and making the insulin work better.
Leave a Reply